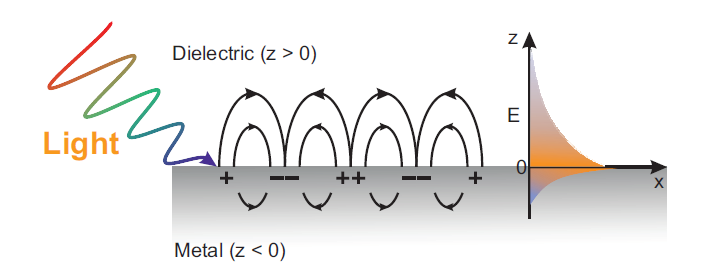
Surface plasmon polaritons (SPP), as an optical surface wave mode with the ability of breaking the traditional optical diffraction limit, can provide not only nanoscale spatial resolution, but also tremendously enhanced local electromagnetic (EM) field. When light reaches a metal-dielectric interface perforated with periodic subwavelength grating structures, surface waves can be generated due to the specific interaction between incident photons and mobile surface electrons. The free electrons inside the metal respond to such external stimulation by performing a resonant oscillation, which consequently generates a type of surface waves at metal dielectric interfaces. Noteworthy, these kinds of charge density oscillations in the metal are named surface plasmons whereas the combined modes between the electromagnetic field and the surface plasmons are denoted as surface plasmon polaritons (SPP). SPP can be utilized for the implementation of highly integrated and efficient nanoscale photonic devices, but also show broad application prospects in optical storage, photocatalysis, and biosensors.
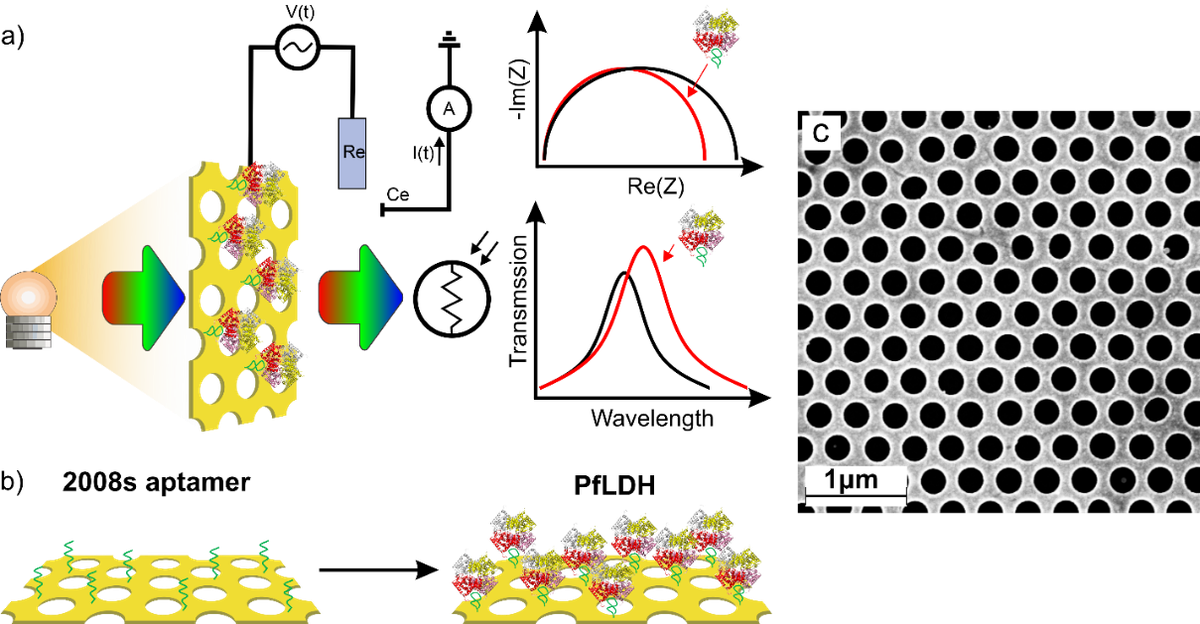
Generally, the optimal operation of every transducer is linked to certain measurement constraints, which limit the accessible concentration interval to the linear dynamic range of detection of the target analyte. These constraints are correlated for instance with the intrinsic signal to noise ratio, the optical penetration depth, and the sensitivity. Furthermore, a degradation of the transducer can be misinterpreted as an authentic response of the target, generating false positive signals (e.g. degradation of redox probes for electrochemical transducer). To eliminate these pitfalls and to extent the accessible concentration range, a sensor can be created which utilizes different transducer principles for the same receptor system in situ. If these transducers possess different dynamic detection ranges then the analyte can be detected in a larger concentration window. Furthermore, two complementary signals are obtained increasing the reliability of the sensor output.
Our group have developed an easy to implement protocol which combines two transducer principles in one aptamer biosensor by simultaneously performing electrochemical (EC) and surface plasmon polariton (SPP) detection based on gold nanoholes arrays. A thin gold film perforated with nanohole arrays is modified with small and highly charged aptamer receptors (aptasensors) and has successfully utilized for the detection of Plasmodium falciparum lactate dehydrogenase (PfLDH), a main biomarker of malaria infections. The nanohole array Au film can be simultaneously utilized as electrode for electrochemical experiments and facilitates the recording of the SPP peak shift of the transmitted light correlated with analyte binding. Such detection principle can lead to an extended dynamic detection range beyond the limit of the electrochemical detection restricted more to the surface binding events.
Dr. Dirk Mayer
Tel.: +49-2461-61-4023
e-mail: dirk.mayer@fz-juelich.de
Publications:
Minopoli, A., Scardapane, E., Ventura, B. D., Tanner, J. A., Offenhäusser, A., Mayer, D., Velotta, R. (2022). Double-Resonant Nanostructured Gold Surface for Multiplexed Detection. ACS applied materials & interfaces, 14(5), 6417-6427.
Minopoli, A., Della Ventura, B., Campanile, R., Tanner, J. A., Offenhäusser, A., Mayer, D., Velotta, R. (2021). Randomly positioned gold nanoparticles as fluorescence enhancers in apta-immunosensor for malaria test. Microchimica Acta, 188(3), 1-9.
Minopoli, A., Della Ventura, B., Lenyk, B., Gentile, F., Tanner, J. A., Offenhäusser, A., Mayer, D., Velotta, R. (2020). Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nature communications, 11(1), 1-10.
Lenyk, B., Figueroa‐Miranda, G., Pavlushko, I., Lo, Y., Tanner, J. A., Offenhäusser, A., Mayer, D. (2020). Dual‐Transducer Malaria Aptasensor Combining Electrochemical Impedance and Surface Plasmon Polariton Detection on Gold Nanohole Arrays. ChemElectroChem, 7(22), 4594-4600.
Hondrich, T. J., Lenyk, B., Shokoohimehr, P., Kireev, D., Maybeck, V., Mayer, D., Offenhäusser, A. (2019). MEA recordings and cell–substrate investigations with Plasmonic and transparent, tunable holey gold. ACS applied materials & interfaces, 11(50), 46451-46461.
