Molecular Electronics
Functionalized Carboxylic Acids
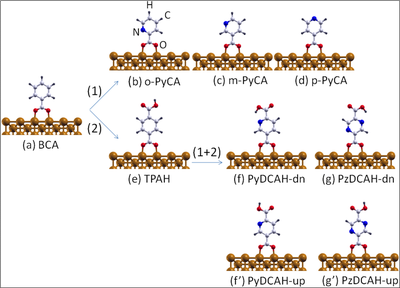
Ball-and-stick model of (a) the benzoate (C7H5O2, denoted also as BCA) molecule chemisorbed on the Cu(110) surface through acarboxylate group (COO). Path (1) illustrates how to functionalize this molecule by substituting a CH group through a nitrogen atom leading to (b) ortho-, (c) meta- and (d) para- pyridine-carboxylate (-(C6H4N(2)O2), denoted also as PyCA). Another possible functionalization path (2) is given by the substitution of an H atom of the aromatic ring through a carboxylic group (COOH) resulting in (e) terephthalate (C8H5O4, denoted also as TPAH). Starting from (e) and further replacing a CH group by one or two N atoms results in (f) dehydrogenated pyridine-2,5-dicarboxylic acid (C7H4NO4, denoted also as PyDCAH-dn) and (g) dehydrogenated pyrazine-2,5-dicarboxylic acid (C7H4NO4, denoted also as PzDCAH-dn).
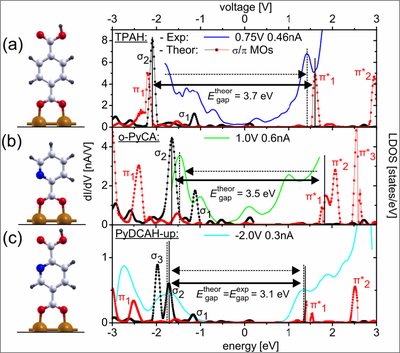
The dI/dV spectrum of (a) TPAH, (b) ortho-PyCA and (c) PyDCAH chemisorbed on the Cu(110) surface together with the calculated local density of states (LDOS). The experimental gap decreases starting from TPAH to PyDCAH-dn in good agreement with the calculated LDOS.
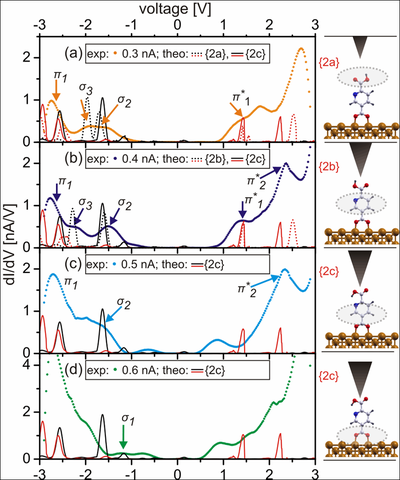
Experimental dI/dV spectra acquired over PyDCAH on Cu(110) with varying current set points at Vset -2.0 V and calculated LDOS with the σ and π orbitals marked in black and red, respectively. With increasing set point current (a)–(d), the σ3 orbitals (marked in light green) change their energetic position relative to σ2 and π1 indicating configurational changes. Outlined in the schematic plots on the right are the molecular parts detected as a function of tip height.
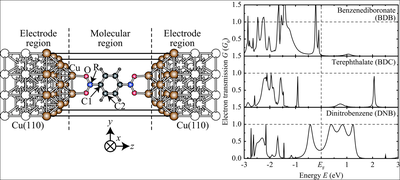
The chemical functionalization of the anchoring groups was performed starting from the carboxylate group by replacing the C atom with a less electronegative B atom (boronate group) and with a more electronegative N atom (nitro group). Although the electronic states of the B, C, and N atoms have a small weight around the Fermi energy, their different electronegativity strongly affects the energetic position of the hybrid states with π character at the aromatic ring. This difference is directly reflected in the electron transmission spectra.
References:
6. Tsukamoto, S.; Caciuc, V.; Atodiresei, N.; Blügel, S.; “Tuning the electron transport of molecular junctions by chemically functionalizing anchoring groups: A first-principles study”, Physical Review B 2012, 85, 245435.
5. Lennartz, M. C.; Atodiresei, N.; Caciuc, V.; Karthäuser, S.; “Identifying Molecular Orbital Energies by Distance-Dependent Transition Voltage Spectroscopy”, The Journal of Physical Chemistry C 2011, 115, 15025.
4. Caciuc, V.; Lennartz, M. C.; Atodiresei, N.; Karthäuser, S.; Blügel, S.; “Fine tuning of the electronic structure of π-conjugated molecules for molecular electronics”, Nanotechnology 2011, 22, 145701.
3. Lennartz, M. C.; Caciuc,V.; Atodiresei, N.; Karthäuser, S.; Blügel, S.; “Electronic mapping of molecular orbitals at the molecule-metal interface”, Physical Review Letters 2010, 105, 066801.
2. Lennartz, M. C.; Atodiresei, N.; Müller-Meskamp, L.; Karthäuser, S.; Waser, R.; Blügel, S.; “Cu-adatom-mediated bonding in close-packed benzoate/Cu(110)-systems”, Langmuir 2009, 25, 856.
1. Atodiresei, N.; Caciuc, V.; Schroeder, K.; Blügel, S.; “First-principles investigation of terephthalic acid on Cu(110)”, Physical Review B 2007, 76, 115433.
( N. Atodiresei )
Arylthio-Substituted Coronenes
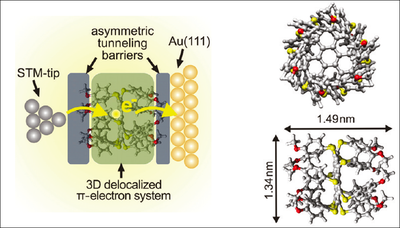
The methoxy groups efficiently decouple the three-dimensional aromatic system of dodecakis-(p-methylphenylthio)-coronene (DMPTC) from the Au(111) surface in this adsorption configuration and facilitate the observation of single electron tunneling effects through the physisorbed molecules. The paramount advantage of our DMPTC molecule Au(111) system is that a double-barrier tunnel junction is constructed without the need of an additional insulating layer.
References:
2. Kowalzik, P.; Atodiresei, N.; Gingras, M.; Caciuc, V.; Raimundo, J.-M.; Blügel, S.; Waser, R.; Karthäuser, S.; “Arylthio-substituted coronenes as tailored building blocks for molecular electronics”, Physical Chemistry Chemical Physics 2012, 14, 1635.
1. Kowalzik, P.; Atodiresei, N.; Gingras, M.; Caciuc, V.; Blügel, S.; Waser, R.; Karthäuser, S.; “Single Electron Tunneling through a Tailored Arylthio-coronene”, The Journal of Physical Chemistry C 2011, 115, 9204.
( N. Atodiresei )
