External Fields
Table of Contents
Shear Flow
Shear flow applied to dispersions of (bio-)macromolecules affects microstructure order, induces new phases, and gives rise to instabilities. Various systems of anisotropic colloids (like platelets and rods), both in the fluid- and glassy- state, as well as red blood cells are of interest.
Red Blood Cells under Flow
Rouleaux are the cylindrical structures that spontaneously form in our blood when there is no flow and which cause our blood to have a high yield stress. In order to understand the flow of our blood it is therefore of fundamental importance to characterize the build up and break up of these rouleaux. We investigate using a fundamental physical-chemical approach to tune interactions this effect as well as our home-build zero-velocity plane shear cell to create ideal flow conditions and 3D microfluidics to create physiological flow conditions [Korculanin2021].
The Molecular Origin Underlying Shear Thinning of Polymers
To resolve the microscopic origin of the shear thinning mechanism in polymeric systems, we perform confocal microscopy under flow of F-actin of several tens of microns long. This allows to probe the time-resolved configuration of the polymer chains in full 3D. We find, for example, that shear thinning is accompanied by a layering of the chains in planes perpendicular to the vorticity direction [Kirchenbuechler2014].
Shearing Nematic Platelets
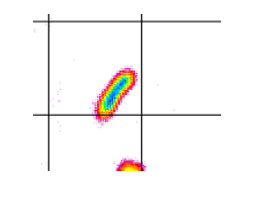
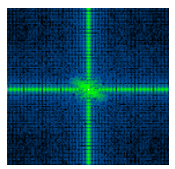
Most soft matter materials cannot be classified as fluids or solids because they possess a dual character: they can have a response that is solid-like or fluid-like, depending on the mechanical deformation. This dual, visco-elastic, character can also be found in an important class of soft matter systems, namely liquid crystals. Liquid crystals can be formed by small ‘colloidal’ platelets, such as clay particles, which all have similar orientation, so that they are in a nematic phase despite of their Brownian motion, which makes them colloidal. We subjected these dispersions of platelets to an oscillating shear field, squeezing the dispersions between two moving plates. By combining this geometry with time-resolved X-ray scattering, we uncover the structural origin of the transformation between solid and liquid-like behaviour and in a cascade of complex flow responses. These new insights might change the view on the origin of visco-elastic behaviour in complex fluids [Korculanin2021,Chen2021].
Rods under Shear
Dispersions of colloidal rods can show very complex behavior under shear flow, depending on the concentration regime. While nematic as well as the dilute suspensions have received a lot of attention, the simple shear thinning response in the semidilute case is surprisingly neither well studied nor understood, even though this is the most very relevant range in biology and industrial application. We gain insight in this behavior be combining biochemistry, producing a library of filamentous bacteriophages with different length and stiffness, 3D rheo-SANS from two different directions, providing 3D particle distributions under shear flow, and elaborated theory [Lang2019].
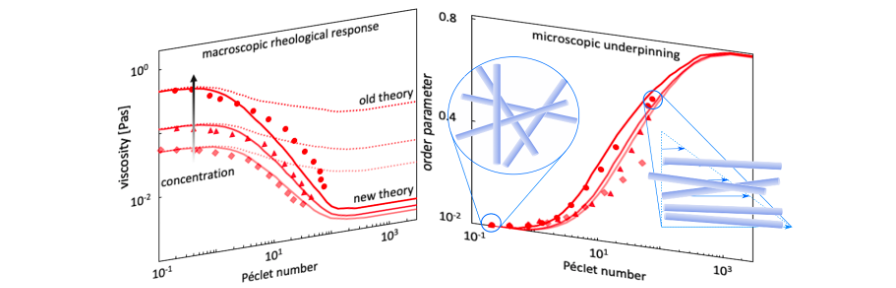
Non-uniform Flow of Glasses
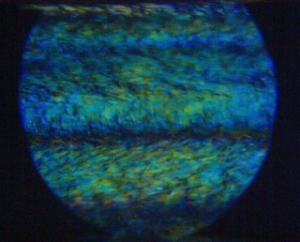
Spatial gradients in the shear rate lead to mass transport, giving rise to non-uniform, banded flow profiles. This instability (the “Shear-gradient Concentration Coupling instability”) is especially relevant for colloidal systems with a yield stress, like glasses and gels. A microscopic theory is developed to explain the origin of the shear-gradient induced mass transport [Jin2014]. Experiments are performed on glasses consisting of colloidal rods which reveal various types of non-uniform flow profiles, amongst which are internal fracture, plug flow, and vorticity banding, as well as banded profiles. It is argued that the shear-concentration coupling instability occurs only for colloidal glasses consisting of particles with short-ranged interactions. The shear-banding instability in our soft rod-glasses is due to the classic shear-banding instability, resulting from strong shear thinning [Dhont2017].
Confinement
In the ultimate vicinity to confining walls, the interaction field of suspended colloidal particles is different from their bulk behaviour, and their dynamics is slowed down as compared to the bulk diffusion [Lang2016]. Besides their scientific interest, these effects are important in many technological, biological and bio-medical processes, like the motion of proteins in membranes, or the approach of a virus or a drug carrier to cells.
Particle Wall Interactions
Using total internal reflection microscopy (TIRM) we can determine the interaction energy profiles between colloidal particles and flat wall with an unprecedented force resolution. Besides investigating theses energies in equilibrium suspension [July2010], we investigate how external fields like shear can be used to tune them. Typical examples are depletion forces induced by anisotropic particles, which can be suppressed by aligning the particles in a flow field [July2012]. More recently, we are developing methods to reliably extract the particle’s Brownian dynamics normal to the wall from TIRM data [De Sio2018]
P. Lang
Near Wall Particle Dynamics
We study the effect of solid surfaces on colloidal dynamics by evanescent wave dynamic light scattering. In very dilute suspension of hard spheres, there is a pronounced slowing down of the particles’ dynamics close to the wall. We investigated the variation of this effect with particle concentration [Liu2015], particle interaction [Liu2016] and particle morphology [Rivera-Morán2021] Current research is concerned with the dynamics of protein coated particle in the vicinity of supported lipid bilayers, as a first step towards a model system for proteins close to cell membranes.
P. Lang
Temperature Gradients
Thermophoresis, also known as the Ludwig−Soret effect or thermodiffusion, is the migration of particles or molecules induced by a temperature gradient. It has been utilized to monitor biochemical reactions and to determine equilibrium constants, which are essential for the development of new pharmaceuticals. Thermophoresis is also discussed in the context of the origin of pre-biotic molecules. The microscopic mechanism of this effect is still not completely understood, especially in systems with hydrogen bonding.
The early-earth formation of pre-biotic molecules
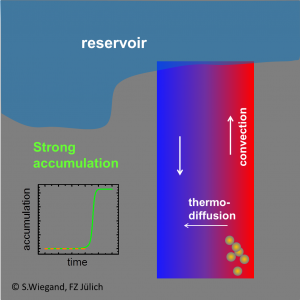
We showed that prebiotic nucleobases can be formed in hydrothermal pores, through a significant accumulation of formamide resulting from a combination of thermophoresis and convection. Starting from typical low concentrations under early earth conditions formamide accumulates at the bottom of hydrothermal pores in about 45–90 days to high concentrated formamide solutions. The conclusion from these findings is that the combination of thermophoretic mass transport and convection is the missing link, which makes the synthesis of prebiotic nucleobases in porous rocks in contact with shallow lakes under early-earth conditions possible [Niether2016, Niether2017]. Click here for the movie.
S.Wiegand
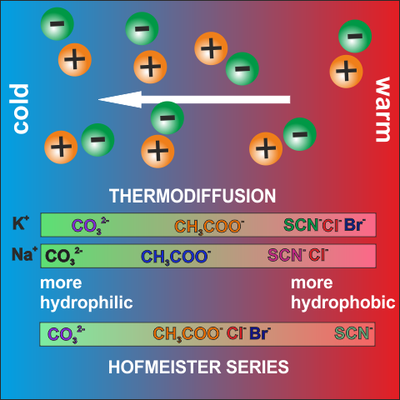
Using thermodiffusion as sensor for ion specific effects
Ions are crucial in biological information processes. Since the pioneering work of Hofmeister, it is known that most aqueous physicochemical processes not only depend on ion concentration and valency, but also on the ion type leading to different water network structures and biological function. Due to the high sensitivity of thermodiffusion to structural changes in the water network we can characterize the ion specific interactions such as the strength hydration of layers formed around the ions [Mohanakumar2021, Mohanakumar2022]. This information helps to develop better models for the biological functions of ions.
S.Wiegand
Understanding protein-ligand binding
Molecular recognition via protein–ligand interactions is of fundamental importance to most processes occurring within living organisms. Structural fluctuations and conformational motions of proteins are essential for the binding of ligands and other interaction partners. It turns out that the thermophoresis is very sensitive to this binding event, but the cause is still unclear. Performing systematic thermophoretic measurements in combination with neutron scattering experiments and isothermal titration experiments we gain a deeper insight into entropic and enthalpic changes of the protein, protein-ligand complex and accompanied hydration layer upon binding [Sarter2020, Niether2020].
S.Wiegand
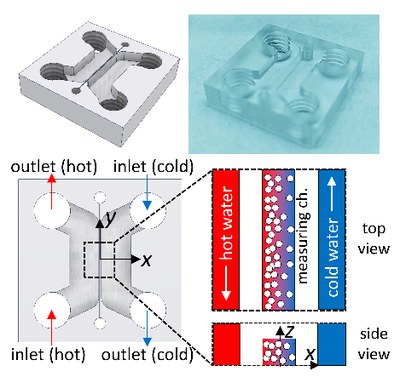
Development of a thermophoretic microfluidic cell
Traditional methods to measure thermophoresis consume large sample volumes, are limited to binary mixtures, and give only indirect access to the applied temperature profile [Lee2020]. We develop a thermophoretic microfluidic cell for quantitative measurements of the thermophoretic quantities of biomacromolecular compounds [Lee2022]. The actual microscale measuring channel lies between cooling and heating channels to achieve a one-dimensional temperature gradient. Fluorescence lifetime imaging microscopy with Rhodamine B is utilized to measure the spatial temperature profile in the channel. Various geometries and materials are optimized to avoid convection and to obtain reliable measurements of complex systems such as proteins.
S.Wiegand
Electric Fields
Electric fields affect microstructural order of macromolecular suspensions, and can induce dynamical states and new phases. We are particularly interested in the response of charged macromolecules to low-frequency fields.
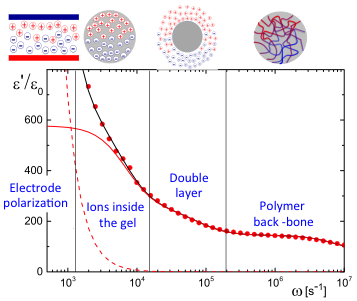
Dielectric spectroscopy of microgel particles
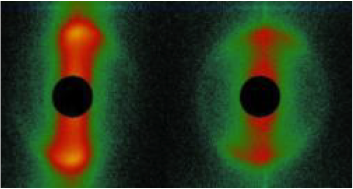
We proposed a theory for the electric polarization due to mobile ions within the polymer network of PNIPAM microgel particles, as well as a theory for electrode polarization that is valid for all electric-field frequencies. Applying these theories to dielectric spectroscopy experiments allowed for the determination of the concentration dependence of the size, the net charge, as well as the degree of ion-association to the microgel PNIPAM polymer back-bone, which quantities are difficult to obtain by other methods. The dielectric spectrum reveals various polarization mechanisms, as shown in the figure: at low frequencies electrode polarization dominates, followed by the polarization relaxation due to mobile ions within the polymer network of the microgel particles, a relaxation due to the electric double layer surrounding the particles, and the polarization relaxation of the PNIPAM polymer network [Mohanty2016]
G. Nägele
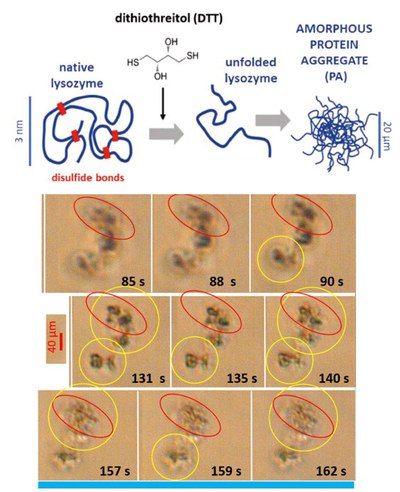
Protein aggregation in weak AC electric fields
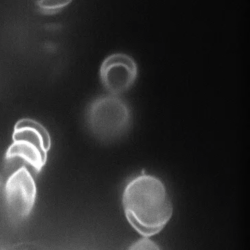
Flexible, amorphous, micron-sized protein aggregates of lysozyme form, depending on the concentration of the added denaturant Dithiothreitol. The pre-formed amorphous protein aggregates are exposed to an externally applied weak alternating current electric field. The denaturant concentration and the frequency and field-strength are systematically varied. The field response is followed in situ by a long time-resolved polarized optical microscopy, revealing field-induced deformation, reorientation and enhanced polarization as well as the disintegration of large aggregates.
Here, the right images are shown for few localized times [Kang2022].
Electric-field induced (transitional) polarization states, localized dynamics, and orientational motion
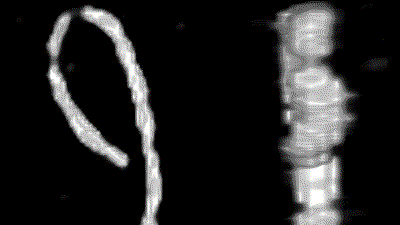
An electric field changes the interactions between molecules in different ways: the form of a single molecule and aggregates of molecules may be affected due to stresses that arise from interactions of charge residues with the field, the electric double layer will be polarized, and field-induced orientation favors interactions between aligned molecules. This can lead to aggregation or the dissolution of aggregates. As an example, the upper movie on the right shows the response of an aggregate of graphene oxide (size is ca. 4 micron) to an electric field (10 kHz and 10 V/mm), and the lower movie shows the Fourier transformed video, which can be analyzed to distinguish the evolution on different length scales.
Both the orientational and translational motion of the aggregate are affected by the external electric field. Since the structures are birefringent, the orientational motion as well as the changes of internal structure are most conveniently probed by depolarized light microscopy. The various field-induced features are studied as a function of frequency and field-amplitude [Kang2021].
K.Kang
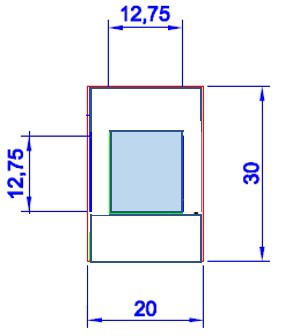
2nd generation of a thin ITO-coated glass cell, adapted to higher electric field strengths
Physiological salt concentrations are sufficiently high that heating becomes an issue already at higher field strengths and frequencies. To avoid heating on applying an electric field, the gap between the electrodes must be diminished. In addition, some of the proteins are available only in small quantities, which also requires such smaller electric sample cells. The cells that are now available have gap-widths ranging from 3 to 100 micron (for example, ca. 10 mHz- 100 kHz and 1-2 V/um).
As for the first-generation cells, the cells convey the electric field by Indium tin oxide (ITO) layers. The cells are transparent, which allows to perform optical microscopy, light scattering, and X-ray spectroscopy, in order to study the field-induced aggregation/disaggregation and phase behavior of various types of proteins and their mixtures in detail.
K.Kang