Protein Aggregation and Phase Behaviour in Electric Fields
About
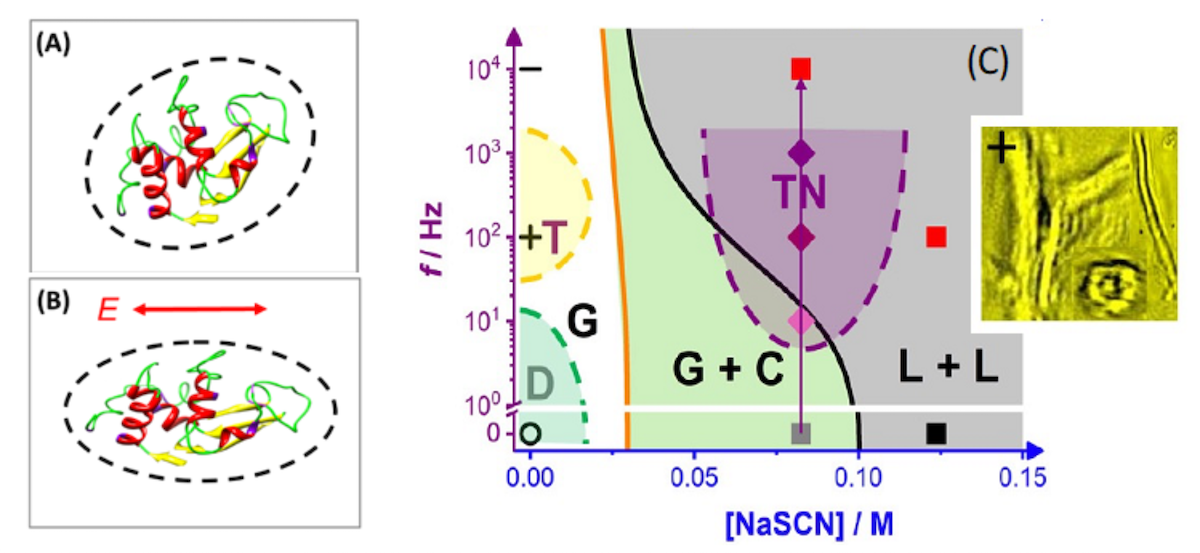
My research fields are focused on collective phase behaviors of charged DNA-viruses (fd) in both non-equilibrium (electric-field and shear-flow) and equilibrium, together with developments of novel scientific instrumentation, light scatterings and image-time correlation spectroscopy. Emergent experimental results have been provided semi-empirical theories on charged species, in particular, low-frequency driven dynamical states of charged DNA rods, field-inducedmicroscopic dynamics and critical slowing down behaviors (glass phenomena) as well the flow response of soft rod-glasses with 3d bulk pattern formations. In the equilibrium phase diagram of charged DNA-rods at low ionic strengths, the orientation kinetics of charged DNA-rods are explored by image-time correlations, which also applied to other interested materials (T4 DNA, lysozyme with antagonistic salt, cellulose nano-fibers/crystals, etc). As ongoing research interests, the protein phase behaviors and amorphous protein aggregations in weak electric fields are to revealed by roles of dissociation constant for condensed ions in both non-equilibrium and equilibrium. To which extent can protein aggregation be inhibited/enhanced by electric fields (also in mixtures of different proteins)? The main topic is how the electric field-induced change of the electrical double layer affect on the structure of proteins (exposure of hydrophobic groups).
Research Topics
- The effect of electric fields, shear flow, and confinement on the dynamics and self-assembly as well as field-induced instabilities of suspensions of anisotropic nano-particles.
- Protein aggregation and phase behaviour in electric fields.
Members
DFG Einzelantrag “Protein phase behaviour in electric fields“, with the HHU (Dr. Florian Platten).

