Amphiphiles
Amphiphilic Block Copolymers in Bicontinuous Microemulsions
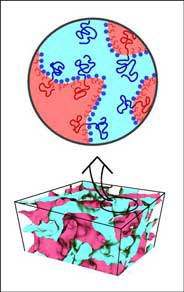
When a small amount of surfactant molecules is added to a phase-separated mixture of oil and water, a homogeneous "microemulsion" phase forms, which contains roughly equal amount of oil and water. This phase has a "bicontinuous" structure, since it consists of two mutually intertwined networks of oil- and water channels (see figure).
Small amounts of an amphiphilic block copolymer lead to a dramatic increase in the volumes of oil and water, which can be solubilized in a bicontinuous microemulsion. The effect of various block copolymers lengths and concentrations on the structure and phase behavior of ternary microemulsions has been investigated. High-precision neutron scattering experiments provide clear evidence that the polymers form uniformly distributed "mushroom" conformations on the surfactant membrane (see figure). Based on these observations, we propose an universal mechanism for the swelling behavior, which is due to the variation of the membrane curvature elasticity.
Cubic bicontinuous phases in ternary amphiphilic systems
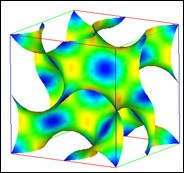
Interfaces in amphiphilic systems can often be well described by elastic sheets with bending rigidity k, saddle-splay modulus k, and spontaneous curvature c0. The amphiphilic monolayers in ternary mixtures with water and oil can arrange in different ways to form micellar, hexagonal, lamellar and various triply periodic, bicontinuous cubic phases. The relative stability of the latter phases can be explained by the way in which their universal geometrical properties conspire with the concentration constraints. We find that the most stable cubic phases with decreasing k<0 are the single and double gyroid structures -- see figure for gyroid minimal surface.
