Carbon Nanofibres as CO2 Catchers
Jülich experts have developed a cost-effective material that efficiently removes carbon dioxide from industrial waste gases. To fabricate this material, the scientists use a technology that is over a hundred years old.

Ansgar Kretzschmar steht vor einem Labortisch und deutet auf den durchsichtigen Würfel darauf, etwa so groß wie eine Waschmaschine: „Der Ansgar Kretzschmar is standing in front of a laboratory bench and pointing towards the transparent cube on it. It’s about the same size as a washing machine. “It’s made of acrylic glass. Purely a safety precaution. Inside it is where we fabricate our fibre mats under a high voltage of 25,000 volts.” Made of fine synthetic fibres, these fibre mats could help to address one of the greatest problems of our time: anthropogenic climate change.
After all, to limit global warming to 1.5 degrees Celsius, as stipulated in the Paris Climate Agreement, carbon dioxide will have to be partially removed from the atmosphere. To do so, materials capable of selectively binding CO2 in industrial flue gases will be required. Such carbon dioxide catchers are being developed by doctoral researchers Ansgar Kretzschmar and Victor Selmert at the Institute of Energy and Climate Research (IEK-9).

“Needless to say, it makes sense to capture the gas exactly where it occurs in large quantities: in biogas facilities, during refuse incineration, or in cement plants. These are the three most important sources of carbon dioxide, which we will not be able to do away with even after the transformation of the energy sector,” says Victor Selmert.
The CO2 could be separated and – using renewable electricity – transformed into valuable chemicals such as methanol, formic acid, or other platform chemicals. These are basic ingredients that are often used in chemistry in large quantities to synthesize a wide range of special products. This would create a circular process for carbon. The practical implementation of the concept, however, is quite challenging, according to the researcher: “All of these gas streams contain other components in addition to carbon dioxide. To be able to utilize the CO2, it must be very selectively separated.”
Searching for the optimal CO2 catcher
Nitrogen, the main constituent in air, is also present in large quantities in these gas mixtures. They can also contain relatively large quantities of water. And CO2 is not the only gas present in biogas facilities; they also predominantly produce the coveted gas methane.
The two researchers from Jülich began to look for materials that would ideally only bind carbon dioxide and let the other gases pass by. Such CO2 catchers are usually pressed into pellets, which absorb the greenhouse gas like a sponge until they are full. Then, the pure carbon dioxide can be released again – either by temporarily increasing the temperature or decreasing the pressure.
Different materials can be used for this – each with very specific strengths and weaknesses, according to Ansgar Kretzschmar: “There is no all-in-one solution yet. Often, highly ordered, porous materials are used. Examples include mineral zeolites or tailored metal–organic frameworks (MOFs). Both substance classes bind carbon dioxide very selectively, but do not release it easily afterwards. Regenerating the material requires an awful lot of energy. And these substances can be very expensive.”
A much more cost-effective solution is a CO2 catcher based on carbon, such as activated charcoal. But this material also adsorbs other gases in the exhaust gas flow, which reduces the effectiveness of the separation. “Activated charcoal is cheap, stable, and easy to regenerate. We are trying to improve its main disadvantage, namely its low selectivity,” says the researcher.
Feinste Fasern spinnen unter Hochspannung
This is where the high-voltage apparatus under the acrylic glass cube comes into play. The two scientists use it to fabricate the precursor to their CO2 catcher. “This is an electrospinning device,” explains Kretzschmar, pointing to a plastic syringe that is clamped to the table inside a holding bracket. “The syringe contains a dissolved polymer known as polyacrylonitrile. We slowly press the viscous fluid out of the cannula. You can see a small droplet forming at the tip of the needle if you have good eyesight.”
A metal drum spins roughly 15 cm away from the syringe. “This is our collector. The high voltage is applied between it and the cannula. It constantly pulls a little of the polymer solution towards the metal drum. As it is pulled towards the drum, the solvent evaporates and fine synthetic fibres form, which in turn gradually form a dense white mat on the collector.”

Electrospinning in itself is nothing new. The first patent for this method was issued in 1900. However, it was not suitable for the production of textile fibres because the throughput was too low. Electrospinning was first rediscovered around 20 years ago as a nanotechnology method and is now suitable for use on an industrial scale with a high throughput. Victor Selmert: “The polymer fibres in our mat are extremely thin – just 200 nanometres in diameter. This is around 100 times thinner than a human hair.” This means that they have a large surface, which is crucial for adsorption processes.
But before the synthetic fibres can adsorb CO2, they must be converted to carbon. To do so, they are heated to 600 to 1,200 degrees Celsius to ensure that nitrogen and hydrogen are separated in the form of volatile compounds. Because the plastic would simply burn at these temperatures, the conversion must occur in the absence of oxygen. The Jülich researchers therefore perform this carbonization in an inert gas atmosphere. This ultimately leads to the formation of extremely fine fibres that predominantly consist of carbon.
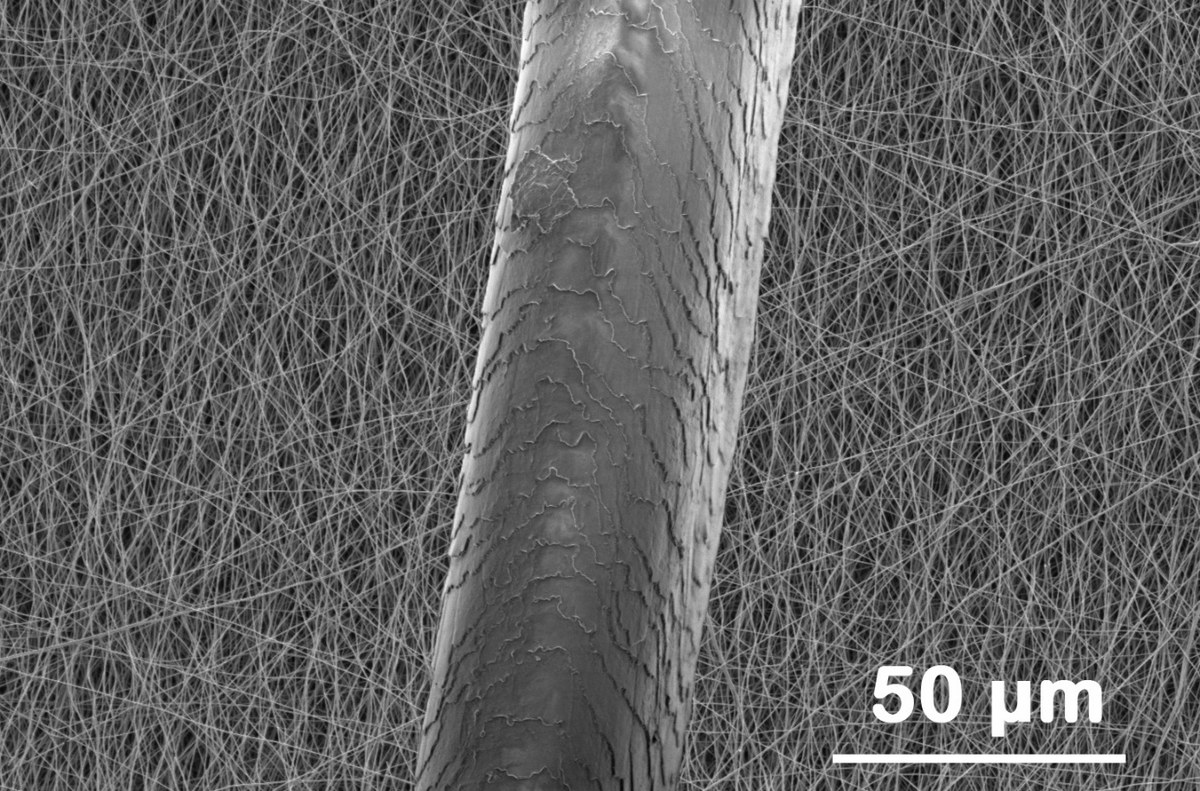
Die Bindung zum CO2: nicht zu stark, nicht zu schwach
“The white fibre mat then becomes black,” says Kretzschmar, taking a Petri dish with a sample of the material out of a drawer. “It almost feels like paper, but it is very fragile.” In this form, it is ideal for use as a selective CO2 catcher that readily releases the greenhouse gas upon regeneration.
“We think this is due to the surface structure of the carbon nanofibres. Grooves can be found along the line of the fibres. The carbon dioxide molecules fit in these grooves and settle with both their ends on the edges. However, they are not bonded as strongly as in other porous materials. This means that they become easily detached from the surface again due to a change in pressure or temperature,” says Kretzschmar.
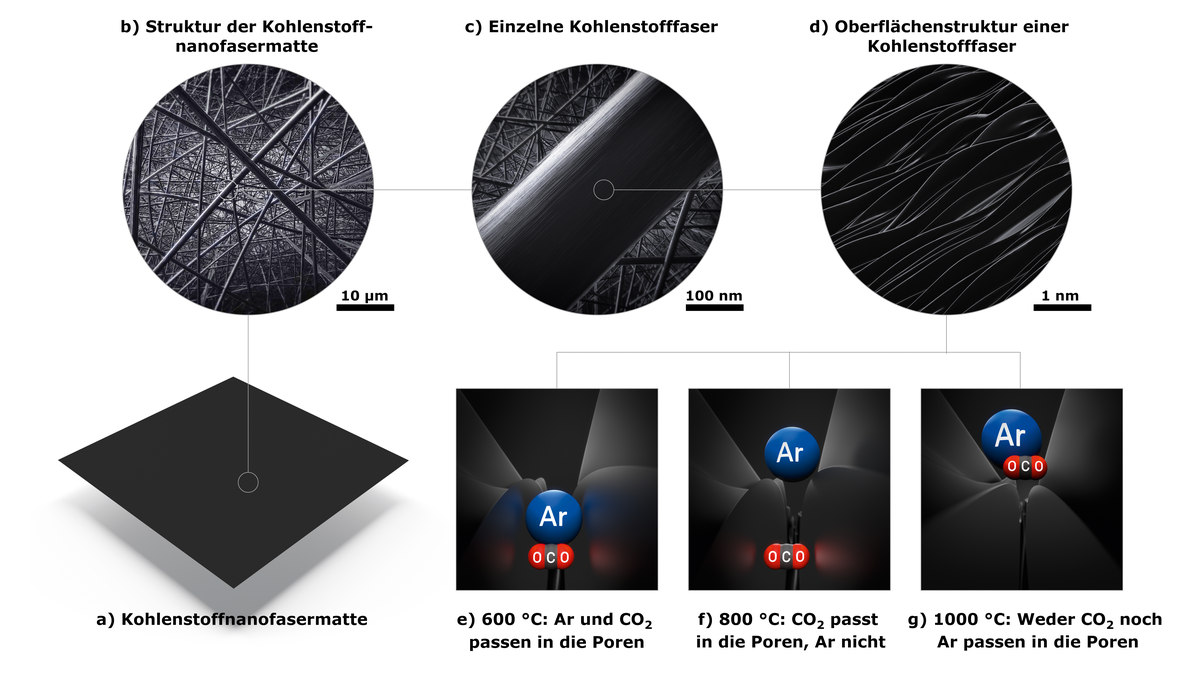
The Jülich researchers are currently also considering the use of the carbon material to convert the separated carbon dioxide into valuable chemicals with the aid of electrolysis cells in which the carbon mats are coated with a catalyst and act as electrodes. As Kretzschmar says: “We have set ourselves the aim of combining the steps of separation and synthesis.”
Arndt Reuning
Further information:
Institute of Energy and Climate Research: Fundamental Electrochemistry (IEK-9)
Original publication: Tailored Gas Adsorption Properties of Electrospun Carbon Nanofibers for Gas Separation and Storage, Ansgar Kretzschmar, Victor Selmert, Dr. Henning Weinrich, Dr. Hans Kungl, Dr. Hermann Tempel, Prof. Dr. Rüdiger-A. Eichel, ChemSusChem, 26 March 2020
DOI: 10.1002/cssc.202000520
